Today, we are delighted to publish “Improving the development and deployment of diagnostics in Southeast Asia”, from our workshop with the Philippine National Academy of Science and Technology (NAST) in Tagaytay City, Philippines.
Diagnostic tests, where available, guide many clinical management decisions around the world. They are key to delivering successful outcomes for both infectious and non-communicable diseases, and offer significant opportunities to improve healthcare provision in low- and middle-income countries.
As part of great global progress in the development of these tests, the Association of Southeast Asian Nations (ASEAN) region has generated many successes, as well as highlighting challenges in this area that need to be addressed.
To help identify ways to address these issues, we held a two-day workshop with the Philippine NAST on 23-24 October 2018.
The workshop formed part of our Global Challenges Research Fund (GCRF) policy work, and brought together approximately 50 scientists, clinicians, policymakers and funders from 10 countries across the ASEAN region.
The forum allowed participants to address the successes and challenges in the development and deployment of diagnostics in the region across five key areas:
- Innovation
- Access
- Regulation
- Industry
- Infrastructure and capacity
Participants identified bottlenecks that could be improved around production and access to products, and the need for better collaboration between disciplines to bring together the necessary range of expertise to develop and improve tests. In many cases, local solutions would be better than adapting existing tests and technology to suit a local need.
They noted how ASEAN researchers and developers can struggle to access samples, and with complex regulation, and how tariffs can increase the cost of technology.
They also face the challenge of hurdling not one, but two ‘valleys of death’ during the development and deployment process.
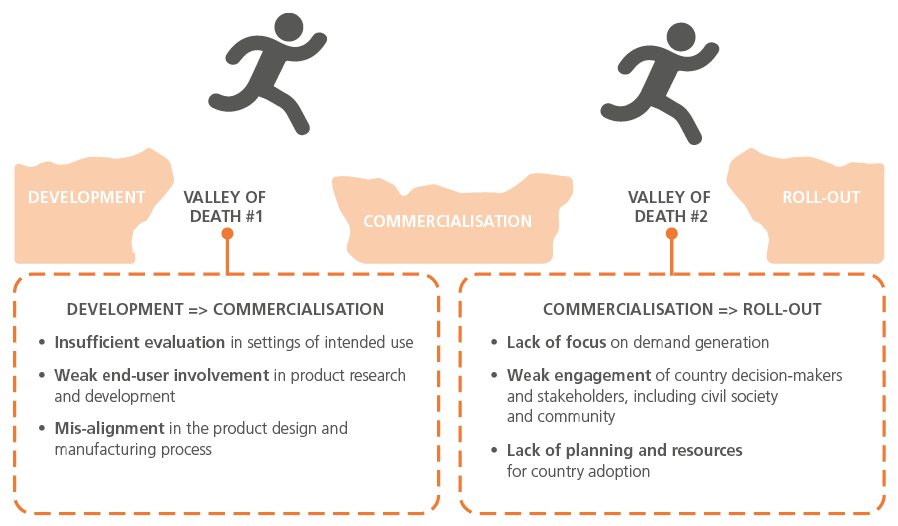
Priority areas to accelerate delivery of diagnostics have been identified as: technology and innovation, partnerships and commercialisation, quality control, and advocacy.
Recommendations include a platform for collaboration across the region, and minimum standards for regulation with a harmonised environment that does not throw up additional country-by-country challenges.
Biobanks can also provide a source for samples and exchange agreements between and among ASEAN countries can stimulate access for the development of locally relevant diagnostic kits.
The NAST will look to build on this workshop and work with ASEAN partners to improve the development and deployment of diagnostics in South East Asia.
For more information, the full report can be downloaded from the right-hand side of this page. For more information about the workshop, please visit our dedicated policy page.
