As one of the signatories of the 2014 Concordant on Openness on Animal Research the Academy of Medical Sciences is committed to transparency around how we conduct, fund or support the use of animals in research.
As part of our ongoing commitment, the Academy publishes yearly statistics on the number of research grants we have funded that propose the use of animals and which species they proposed to use.
The Academy utilises expert peer review in reviewing all of our research grants. In cases where the applications mention the use of animal research, we ensure that the benefits of the research to human and animal health outweigh any potential harm to animals during the research. All research using animals which is conducted in the UK must also be approved by the Home Office.
We send relevant applications to be reviewed by the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs)*. In 2019, we used the NC3Rs peer review service twice; both applications were subsequently recommended for funding at Panel.
The Academy supports the principle that animals should only be used in research when no alternatives exist to find out the same information. We utilise the 3R's to refine, reduce and replace the use of animals in research and our awardees are required to follow ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments) in order to minimize unnecessary studies whilst improving the design, analysis and reporting of animal research to maximise the information published.
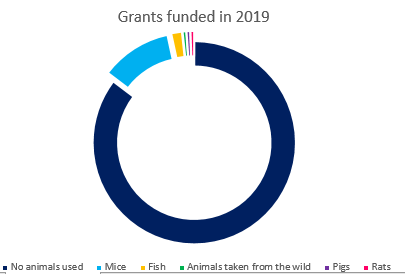
Statistics for 2019:
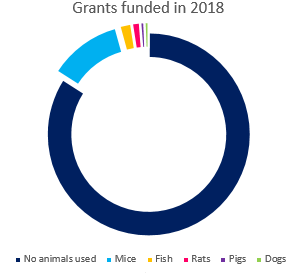
In 2019, out of the 177 grants that the Academy awarded, 26 grants (or 15%) proposed the use of animals in their research**. This compares to a similar proportion in 2018, in which 26 of 163 awards (16%) proposed using animals.
Of those 2019 studies proposing the use of animals:
- 20 of the studies proposed using mice
- 3 proposed using fish
- 1 proposed using rats
- 1 proposed using pigs (this application underwent review by NC3Rs)
- 1 proposed using animals taken from the wild - rodents (this application underwent review by NC3Rs)
* Including applications which propose the use of non-human primates, dogs, cats, equines and pigs.
** Defined as applications for which the proposed use of animals would fall under the ‘Animal (Scientific Procedures) Act 1986’, whether based in the UK or internationally. This act regulates the use of any protected animal in research or scientific procedure which may cause pain, suffering, distress or lasting harm to the animal. Protected animals are defined by the act as any living vertebrate apart from man and living cephalopods.
In 2019, one award involved the use of animals outside of the UK. This application proposed the trapping of rodent urban wildlife (listed above as ‘animals taken from the wild’) for faecal samples. It was referred to NC3Rs for review. This review process ensures that animal welfare standards are genuinely high and exceed the legal minima, and that overseas work is conducted to standards equivalent to those in the UK.
For more information about the Academy's position, please see our Statement on the use of animals in research
For more information on the Concordat, please visit the dedicated UAR website